Average Kinetic Energy Per Molecule of a Gas
OR E 32NKᵦT. From equation of state PV NKᵦT.

The Average Kinetic Energy Per Molecule Equation For An Ideal Gas Ib Physics Youtube
The equipartition theorem says that.

. 617 10 21 k JB. View the full answer. The average kinetic energy of a collection of gas particles is directly proportional to absolute temperature only.
Since the force per collision becomes larger as the temperature increases the pressure of the gas must increase as well. 8 When heat is removed from a sample what happens to the temperature and. Translational kinetic energy of a diatomic gas makes up 35th of its internal energy.
Kinetic Molecular Theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. The Boltzmann constant is 138. The average kinetic energy of an ideal gas per molecule in SI units at.
The last postulate of the kinetic molecular theory states that the average kinetic energy of a gas particle depends only on the temperature of the gas. 2 E 3 2 Nk B T. According to kinetic theory of gases the P exerted by a gas of density rho and rms velocity v is given by.
The average kinetic energy of the molecules in a gas sample depends only on the temperature T. Average KE of translation per unit volume of the gas. The average kinetic energy of a gas molecule can be determined by knowing The average translational energy and the rms.
Speed of molecules in a sample of oxygen gas at 300 K are 621 10-21 J and 484 ms respectively. At lower temperatures the only contribution to kinetic energy is due to the translational motion. Therefore the total energy of the gas is E N m v 1 2 m v 2 Substituting in equation 1 PV 2 3 E.
Here k is boltzmann constant and T is absolute temperature. 617 10 20 k JD. 617 10 21 JC.
However given the same kinetic energies a lighter molecule will move faster than a heavier molecule as shown in the equation for rms speed 3RT rms speed V where R 8314 Jmol-K and M is molar mass in kilograms per mole. 4 Does heating a gas increase kinetic energy. Average kinetic energy 23.
For each quadratic degree of freedom a particle in a system at thermal equilibrium at. The formula for the kinetic energy of a gas defines the average kinetic energy per molecule. EN 32KᵦT is related only to Temperature is independent of pressure volume or nature.
For a classical gas ie. See the answer See the answer done loading. 5 What happens when gas is heated.
716 10 20 J. Therefore NKᵦT 23E. Well away from the quantum and relativistic regimes we can use the equipartition theorem.
Average kinetic energy 23. 7 When a gas is heated all of the absorbed energy is converted to kinetic energy. K average kinetic energy per molecule of gas J.
We know that PVnRT 9 Thus equating equation 8 and 9 we get-. P frac13 rho v2 Mass per unit volume of gas Volume x Density. 12mv²n again 12mv² is the average kinetic energy of each molecule say e so we have 23neP thus putting them in ideal gas equation nw get 23nVe RT no nV is total number of.
Use the following formula for the average kinetic energy of an ideal gas per molecule. 6 When a sample of a gas is heated at constant pressure the average kinetic energy of its molecules does what. The kinetic energy of a molecule in a diatomic gas is as you correctly stated 52 NkT 52 nRT However this is only an approximation and applies in intermediate temperatures.
The average translational kinetic energy per molecule of ideal gas at 47 degree centigrade. From ideal gas equation PV Nk B T 2 3 E. The result above says that the average translational kinetic energy of a molecule in an ideal gas is 32 kT.
The kinetic energy is measured in Joules J and the temperature is measured in Kelvin K. Note- The Boltzmann constant is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. There is also no energy associated with bonds between atoms in molecules because there are no bonds in a monatomic gas.
The average kinetic energy of an ideal gas per molecule is given by the expression. 100 2 ratings average KE per molecule 32kT. According to kinetic theory of gases absolute temperature of a gas is directly proportional to the average kinetic energy of translation per molecule asked Nov 5 2021 in Physics by JaishankarSahu 852k points.
The quantity m v 1 2 m v 2 represents the average translational kinetic energy of an ideal gas molecule. EqE frac 3 2Nk_ bT eq where E is the average kinetic energy of the gas T is the. What is the average translational kinetic energy per molecule of an ideal gas at a temperature of 300 K.
Translational Kinteic Energy Per Molecule ie. This is the best answer based on feedback and ratings. Hence the average kinetic energy of an ideal gas per molecule is 617 times 10 - 21J So the correct answer is option A.
The average kinetic energy of an ideal gas per molecule in SI units at 25 C will beA. Begin array lpfrac mnv 2 3end array. What is the Average Kinetic Energy of a Gas Molecule.
What is the average kinetic energy per molecule of an ideal gas at a temperature of 300 K. You see for an ideal gas PV RT then gain P 13 mnv² where m is mass of each molecule n is called number density and v is the RMS velocity of the molecules now 1 3 mnv² can be rewritten as 23. The following is the deduction of kinetic theory in terms of pressure.
The kinetic energy of the translational motion of an ideal gas depends on its temperature. Boltzmanns constant is k 138.

Average Kinetic Energy Temperature Of A System Video Lesson Transcript Study Com
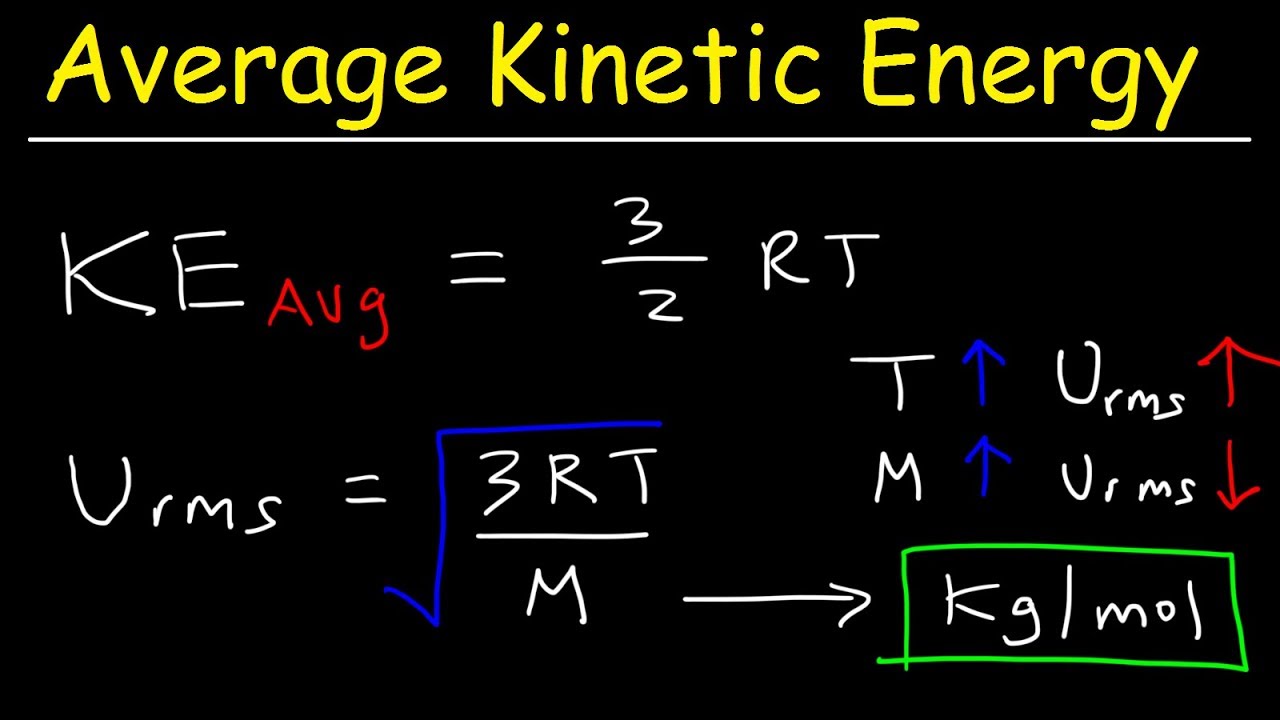
Average Kinetic Energy Of A Gas And Root Mean Square Velocity Practice Problems Chemistry Gas Laws Youtube

The Average Kinetic Energy Per Molecule Equation For An Ideal Gas Ib Physics Youtube
No comments for "Average Kinetic Energy Per Molecule of a Gas"
Post a Comment